Popular posts from this blog
Ionic bond
Definition: Ionic bond is the electrostatic interaction (attractions) which occurs between the positively charged ion and the negatively charged ion . How does it forms: It forms when electropositive element compelety transfer one or two electrons to and electronegative element, in this process one loses electrons (gaining positive charge) and the other gains electron (become negatively charged) , now positive and negative charges attract each other, forming ionic bond. But which element will loses the electrons and which one is the gainer? the elements at the left side of periodic table looses electrons those are the least electronegative elements in the periodic table i.e electropositive. the electronegativity will increase going to the right in the periodic table , and from bottom to top, some the elements at the right sight of the periodic table are the one with highest electronegativity so those are the one who gain electrons becoming negatively charged. In the ...












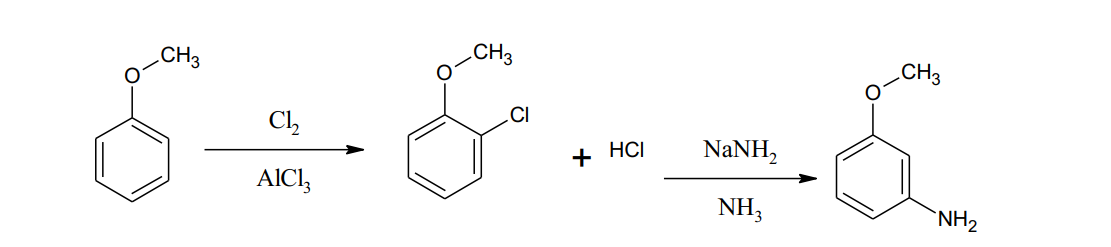


Nice and good work
ReplyDelete